CLICKVIEW 9i Tumor Surveillance Tracking
Voice Activated, Hands-Free Digital Structured Reporting Software
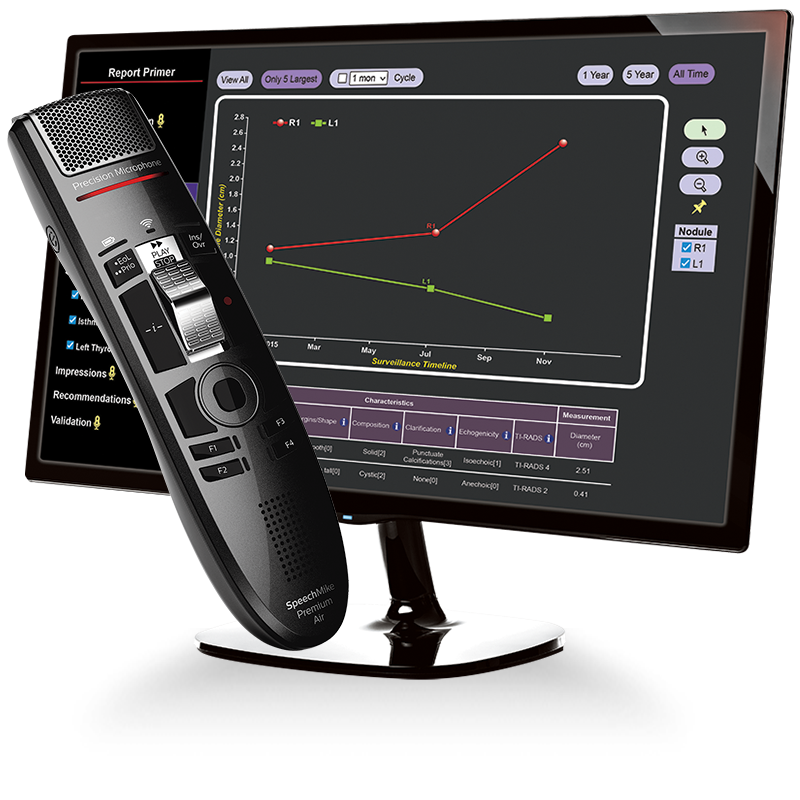
The 9i Innovative Voice Activated User Interface is a Game Changer.
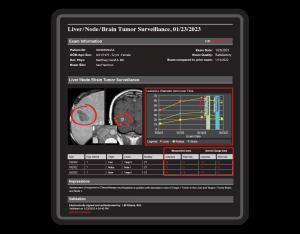
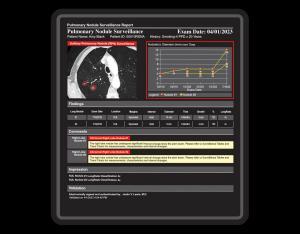
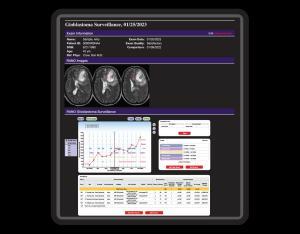
One common characteristic, shared by all suspicious tumor lesions, provides the paramount rationale for long-term radiological surveillance of the potential to threaten life. The unique function of surveillance reports is to document the image and growth features of suspicious chronic lesions over weeks, months, and years.
According to the Centers for Disease Control and Prevention (CDC), 605,213 people died of cancer in the United States in 2022; cancer is the second leading cause of death behind cardiovascular disease, which took 695,547 lives in 2022.
Initial Detection
Eight of the ten most deadly cancers manifest as primary tumors and develop secondary metastases in distant organs. The sizes of the primary tumors and metastases are mostly measurable and used as an index in assessing the response to therapeutic intervention with serial Ultrasound, CT, and MRI studies.
Systematic Surveillance
Systematic radiological surveillance screening is not new. The American College of Radiology has developed an ongoing series of Reporting and Data Systems (RADS) report formats “to provide standardized imaging findings terminology, report organization, assessment structure and classification for reporting and data collection in patient imaging.” The RADS systems seek to reduce the reporting variability with descriptions of image findings, a lexicon, and a structured reporting format. The RADS series, many of which are used routinely in many practices, encompasses reporting for breast (BI-RADS), liver (LI-RADS), thyroid (TIRADS), lung (LUNG-RADS), and others.
However, a similar standardized, systematic reporting protocol for surveillance of non-RADS reporting over timelines extending from weeks, months, and years that automatically compares prior and current reports for chronic lesions is infrequent. Although necessary, it has historically been a slow and cumbersome process, requiring manual comparison of prior studies and entered data.
However, CLICKVIEW 9i changes that.
CLICKVIEW 9i Surveillance database vastly improves tumor surveillance by:
- Reducing the radiological reporting process, including review of priors, to minutes
- Standardizing the reporting process and user-defined formats (image descriptions, lexicon, report format) over serial exams that may extend over years.
- Automating instant display of past data from prior reports
- Incorporating uncomplicated trend charts, measurements, and impressions
- Incorporating context-sensitive Macro Libraries
- Linking Findings, Impressions, and Recommendations
Therapeutic Intervention and CLICKVIEW 9i Tumor Surveillance Structured Reporting
Following diagnosis and initiation of planned therapy, several protocols are used to assess response to therapeutic interventions by evaluating tumor size, burden, and progression.
CLICKVIEW 9i Tumor Surveillance Tracking Reports support protocols such as ACR RADS guidelines (Lung-RADS, TI-RADS, and others), Response Evaluation Criteria In Solid Tumors (RECIST 1.1), and Response Evaluation in Neuro-Oncology for Gliomas (RANO). However, these systems do not provide the program database capability to compare prior studies to the current study automatically.
CLICKVIEW’s 9i Tumor Surveillance Structured Reports database management system automatically organizes and provides instant access to prior surveillance measurement data and completed reports. The robust graphical multi-media display of CLICKVIEW 9i provides a fast, instantly comprehensible real-time comparison of prior response assessment reports to current data.
Non-Tumor Surveillance of Suspicious lesions.
CLICKVIEW 9i provides instant comparisons for surveillance of suspicious lesions in multiple organ systems. These lesions also share the critical characteristics of threatening life, although in different settings and ways:
- Aortic Abdominal Aneurysms (AAA)
- AV malformations
- Calcified incidental findings
- Osteoporosis
There is a reason we are referred to as
“The radiology structured reporting company”
35+
Years Structured Reporting Experience
25
Million Reports
20+
Years Web Architecture
~2.5
~1,500
Active Weekly Users